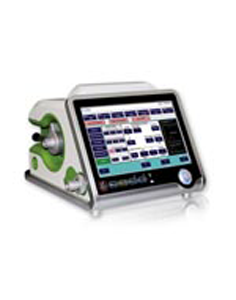
 Ventilator, a Machine to decrease respiratory problems
Ventilator, a Machine to decrease respiratory problems
Nowadays, due to the industrialization of societies, the percentage of respiratory problems has increased. The lung is the internal organ most vulnerable to infection and injury from the external environment because of its constant exposure to particles, chemicals and infectious organisms in ambient air.
Globally, at least 2 billion people are exposed to the toxic smoke of biomass fuel, typically burned inefficiently in poorly ventilated indoor stoves or fireplaces. One billion people inhale polluted outdoor air, and 1 billion are exposed to tobacco smoke. Although respiratory impairment causes disability and death in all regions of the world and in all social classes, poverty, crowding, environmental exposures and generally poor living conditions increase vulnerability to this large group of disorders.
Respiratory diseases impose an immense worldwide health burden. About 3 million people die each year, making it the third leading cause of death worldwide and the numbers are increasing. About 334 million people suffer from asthma, which is the most common chronic disease of childhood, affecting 14% of children globally.
Different types of ventilators:
- The ventilator is used in ICU CCU NICU and emergency departments. Some devices are portable and are powered by air pressure or pneumatics via electricity or batteries.
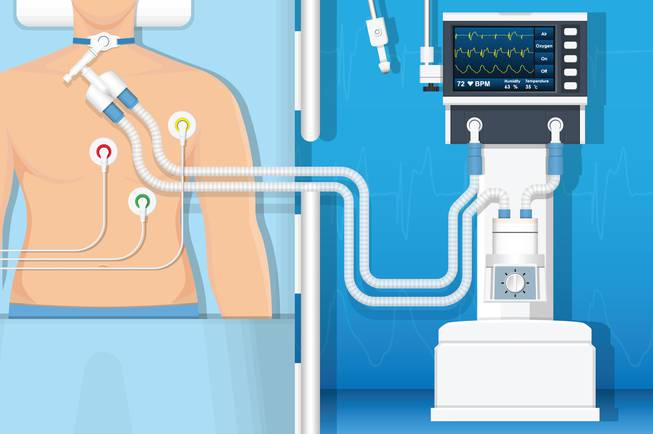
- ICU fans are larger than the portable ones and uses the AC electricity repeatedly, but it has a battery for internal transportation. These devices have displays of respiration rate, patient exhalation volume and carbon dioxide content in exhalation (like capnography’s work)
- In the NICU section, much more accurate ventilators are used because they have to be more careful in low volumes. Ventilators can play a very important role in the respiratory function of premature infants because any negligence in this area endangers the lives of infants.
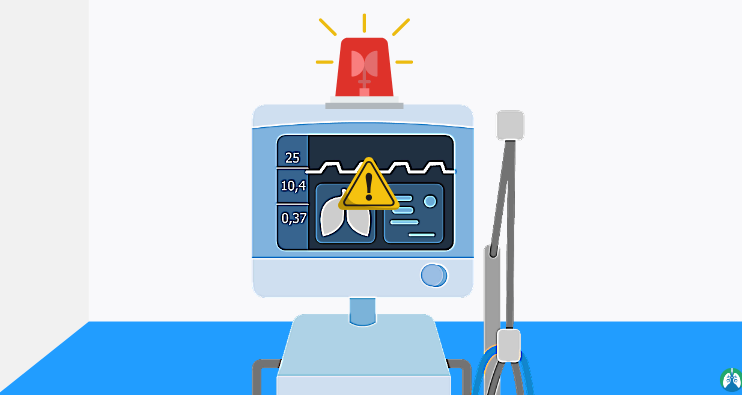
A variety of alarms are installed in the ventilator, such as alarms for decreasing and increasing pressure, oxygen depletion, apnea (respiratory arrest) and problems related to the device that is unable to breathe for any reason, the alarm is activated and to prevent suffocation, the ventilator safety valve opens to use the air of the room. Most ventilators have a system to be connected with their peripherals. The user or operator must be familiar with the correct way to work with the ventilator and its alarms, and to avoid any mistakes, the operator must be properly trained to work with the device. Lung test is used to calibrate the ventilator and anesthesia device.


Saadat Company produced the ventilator in Iran which is founded on 1998 by getting support from a research institute, Jouyandegan Rah Saadat. Saadat produces patient monitors (display size 4.3 to 18.5 inches) equipped with ECG, SPO2, NIBP, IBP, Temp, Resp, Gas Analyzer.
Different models of Monitors are as bellow:
- (Novin S630, S1600, S1800, Zagros Modular, Alborz B5, Alborz B9, Aria, ZAGROSS)
- Central system (Sahand)
- ECG systems with (Dena 350, 650)
- Aria (TC) system with the ability to send life signals on wireless communication platforms in emergency Operations
- Coating equipment with the ability to record patients life signals and transfer data to health centers by smartphones
- Compressor ventilator system can use for ICU to adult and children
Company’s productions are responsive to emergency departments, Outpatient operating room, open heart room, recovery rooms, ICU and CCU in hospitals and clinics, also for taking care of The Saadat company products which have warranty for 2 years and 10 years for services and it has a pleasure for all health center. In 2000, the company succeeded in obtaining the approval of the applied standard of its products by the General Directorate of Medical Equipment also in 2001, the company’s products received the ISO 9001 and EN 46001 international standard certification, it also has international certificates of ISO 13485 and ISO 9001 from QS Institute in the field of design and production of equipment for monitoring vital signs of the patient.


| Technical Features |
| Ventilation modes |
Assisted Control Mandatory Ventilation |
CMV |
| Synchronized intermitted Mandatory Ventilation |
SIMV |
| Spontaneous Ventilation |
SPONT |
| Breath types |
Volume-controlled breaths |
V-CMV, V-SIMV |
| Pressure-controlled breaths |
P-CMV, P-SIMV, PS |
| Volume targeted pressure-controlled breaths (pressure regulated volume control) & (volume support) |
PRVC-CMV, PRVC-SIMV, VS |
| Dual level PEEP breaths (spontaneous positive airway pressure) |
SPAP |
| Patient height |
Cm 250-42 |
| IBW (Ideal Body Weight) |
Kg 200-5 |
| Gender |
Male, Female |
| VBS
(Ventilation Breathing System) |
Acceptable compliance range |
Ml/cmH2O 5- 0.5 |
| Acceptable Inspiratory Resistance Range |
cmH2O.s/l 2.5 -0 |
| Acceptable Expiratory Resistance Range |
cmH2O.s/l 2.5 -0 |
| Apnea backup |
setting |
On(Rate, Control, Ti) off |
| Breath triggering |
Pressure triggering |
cmH2O(20-)-(2-) |
| Flow triggering |
l/min 20-2 |
| Performing procedure |
Inspiration-Hold |
Pause the ventilator at end inspiration
Maximum allowable duration shall be 20 sec |
| Expiration-Hold |
Pause the ventilator in inspiration
Maximum allowable duration shall be 20 sec |
| Manual breathing |
Delivery a mandatory breath during the next expiratory breath in all mode |
| P0.1 |
The maximal slope of the airway pressure drop during the first 100 MS when the airway is occluded |
| NIF |
The maximum negative pressure generated during inspiration against an occluded airway |
| Suction |
This function allows breaths to be delivered with a 100% O2 concentration, before and after a tracheal suction maneuver
S: Resolution: 10 120-20 |
| O2 100% |
Delivers O2 100% for 2 minutes |
| Standby |
Places ventilator in standby mode |
| Sigh |
Breaths delivered at a regular interval, with a higher than normal volume or pressure |
| Nebulizer |
During the nebulization time, the nebulizer valve opens synchronous to inspiration to provide pressure for an external medication nebulizer |
| Alarm system |
Auditory alarm |
Internal speakers |
| Visual alarm |
In the TFT display |
| Indicator |
LED |
| Types |
Technical, physiological, messages |
| priorities |
High, medium, low |
| Physical data |
Width x depth x height (main case) |
X 35 x 29 cm 45 |
| weight |
Kg 12 |
| Noise level |
TBD |
| display |
TFT size |
Wide, with touch screen *12.1 |
| Pressure safety |
Maximum limited pressure |
cmH2O ± 10110 |
| Maximum operation pressure |
cmH2O 80 |
| Compliance and approvals |
Classifications |
Class I equipment, type BF, internally powered, drip-proof equipment for continuous operation
EMC related group and classification: I-B |
| Meets international standards |
EN 60601-1 : 2006
EN 60601-1-2 : 2007
EN 60601-1-8 : 2007
EN 60601-2-12 : 2006 |
 Technical Specification:
Technical Specification:
- Can be used for both adults and children (The minimum current volume is 20 ml)
- Equipped with a 12-inch color touch screen
- Ease of working with the machine due to the suitable physical characteristics
- Automatic adjustment of respiratory parameters by IBW determination
- Has common and advanced models of pressure, volume and volume _pressure
- The Ability to display Curves and respiratory loops and numerical values
- Data retention for 72 hours
- Has adjustable and smart alarms
- The Ability to work with internal battery for 2 hours
- Automatic air leak compensation
- Automatic airway compliance compensation
- Has a pressure and floating trigger
The main use of ventilators is not treating the lung diseases. This machine with mechanical ventilating and supporting the lungs until the problems are solved, supplies the ventilation and oxygenation.
According to the things mentioned above and the importance of the ventilator, the world is currently facing a deficit of this machine, in addition Iran is in shortage of this device too also, due to the fact that this device is produced in most Islamic countries which are the members of D8tten (D-8 Technology and Transfer and Exchange Network), these countries can work together and this synergy in the form of d-8 organization for economic cooperation will enable them to produce qualified and more productions to send them to other parts of the world.

So far, PARDIS Technology Park as the largest technology park in the country with the aim of supporting and investing in the development of technology and innovation units could support more than 250 technology units and knowledge-based in the fields of advanced technologies such as information and communication technology, biotechnology, nanotechnology, new materials and mechanics.
Pooyandegan Rah-e Saadat Company, which produces ventilator products, is one of the companies which is supported by PARDIS Park and also the customers who are going to buy this machine will be supported by PARDIS Park too.

 Ventilator, a Machine to decrease respiratory problems
Nowadays, due to the industrialization of societies, the percentage of respiratory problems has increased. The lung is the internal organ most vulnerable to infection and injury from the external environment because of its constant exposure to particles, chemicals and infectious organisms in ambient air.
Globally, at least 2 billion people are exposed to the toxic smoke of biomass fuel, typically burned inefficiently in poorly ventilated indoor stoves or fireplaces. One billion people inhale polluted outdoor air, and 1 billion are exposed to tobacco smoke. Although respiratory impairment causes disability and death in all regions of the world and in all social classes, poverty, crowding, environmental exposures and generally poor living conditions increase vulnerability to this large group of disorders.
Respiratory diseases impose an immense worldwide health burden. About 3 million people die each year, making it the third leading cause of death worldwide and the numbers are increasing. About 334 million people suffer from asthma, which is the most common chronic disease of childhood, affecting 14% of children globally.
Different types of ventilators:
Ventilator, a Machine to decrease respiratory problems
Nowadays, due to the industrialization of societies, the percentage of respiratory problems has increased. The lung is the internal organ most vulnerable to infection and injury from the external environment because of its constant exposure to particles, chemicals and infectious organisms in ambient air.
Globally, at least 2 billion people are exposed to the toxic smoke of biomass fuel, typically burned inefficiently in poorly ventilated indoor stoves or fireplaces. One billion people inhale polluted outdoor air, and 1 billion are exposed to tobacco smoke. Although respiratory impairment causes disability and death in all regions of the world and in all social classes, poverty, crowding, environmental exposures and generally poor living conditions increase vulnerability to this large group of disorders.
Respiratory diseases impose an immense worldwide health burden. About 3 million people die each year, making it the third leading cause of death worldwide and the numbers are increasing. About 334 million people suffer from asthma, which is the most common chronic disease of childhood, affecting 14% of children globally.
Different types of ventilators:

 A variety of alarms are installed in the ventilator, such as alarms for decreasing and increasing pressure, oxygen depletion, apnea (respiratory arrest) and problems related to the device that is unable to breathe for any reason, the alarm is activated and to prevent suffocation, the ventilator safety valve opens to use the air of the room. Most ventilators have a system to be connected with their peripherals. The user or operator must be familiar with the correct way to work with the ventilator and its alarms, and to avoid any mistakes, the operator must be properly trained to work with the device. Lung test is used to calibrate the ventilator and anesthesia device.
A variety of alarms are installed in the ventilator, such as alarms for decreasing and increasing pressure, oxygen depletion, apnea (respiratory arrest) and problems related to the device that is unable to breathe for any reason, the alarm is activated and to prevent suffocation, the ventilator safety valve opens to use the air of the room. Most ventilators have a system to be connected with their peripherals. The user or operator must be familiar with the correct way to work with the ventilator and its alarms, and to avoid any mistakes, the operator must be properly trained to work with the device. Lung test is used to calibrate the ventilator and anesthesia device.

 Saadat Company produced the ventilator in Iran which is founded on 1998 by getting support from a research institute, Jouyandegan Rah Saadat. Saadat produces patient monitors (display size 4.3 to 18.5 inches) equipped with ECG, SPO2, NIBP, IBP, Temp, Resp, Gas Analyzer.
Different models of Monitors are as bellow:
Saadat Company produced the ventilator in Iran which is founded on 1998 by getting support from a research institute, Jouyandegan Rah Saadat. Saadat produces patient monitors (display size 4.3 to 18.5 inches) equipped with ECG, SPO2, NIBP, IBP, Temp, Resp, Gas Analyzer.
Different models of Monitors are as bellow:


 Technical Specification:
Technical Specification:
 So far, PARDIS Technology Park as the largest technology park in the country with the aim of supporting and investing in the development of technology and innovation units could support more than 250 technology units and knowledge-based in the fields of advanced technologies such as information and communication technology, biotechnology, nanotechnology, new materials and mechanics.
Pooyandegan Rah-e Saadat Company, which produces ventilator products, is one of the companies which is supported by PARDIS Park and also the customers who are going to buy this machine will be supported by PARDIS Park too.
So far, PARDIS Technology Park as the largest technology park in the country with the aim of supporting and investing in the development of technology and innovation units could support more than 250 technology units and knowledge-based in the fields of advanced technologies such as information and communication technology, biotechnology, nanotechnology, new materials and mechanics.
Pooyandegan Rah-e Saadat Company, which produces ventilator products, is one of the companies which is supported by PARDIS Park and also the customers who are going to buy this machine will be supported by PARDIS Park too.